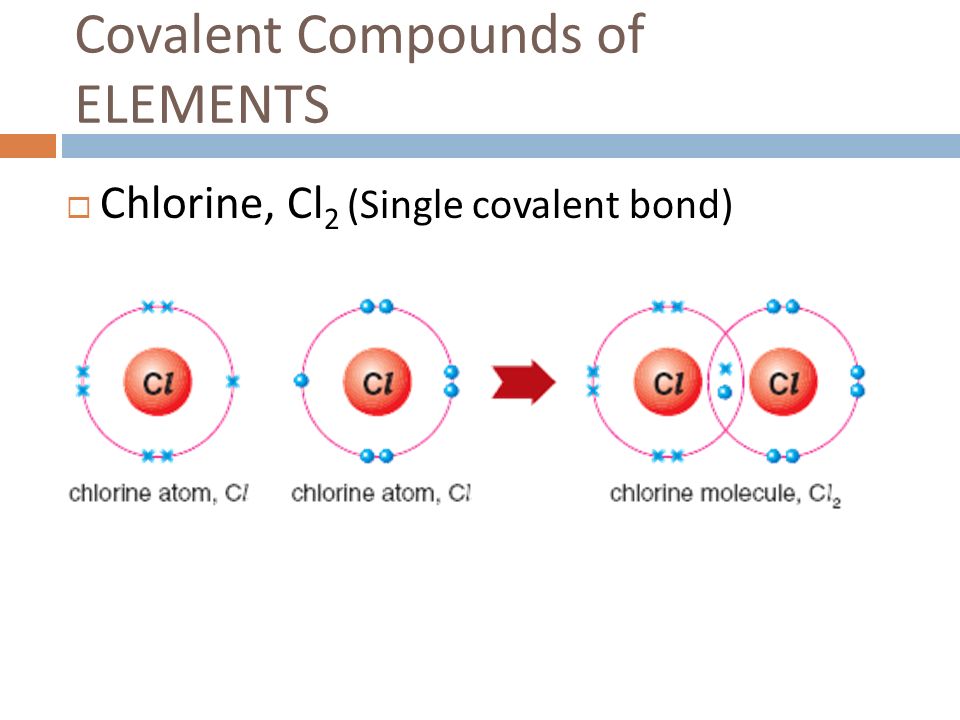
Chlorine Valence Electrons Charge . 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Valence electrons are the electrons in the outermost shell, or energy level, of an atom. Valence electrons = 7, lone pair. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. Chlorine has 7 valence electrons. The 3s^2 3p^5 electrons are the outermost electrons, so chlorine has seven valence electrons. In a picture, the valence. (in table salt, this electron comes from the sodium atom.) e− + cl cl− e − + cl cl −. Only one more electron is needed to achieve an octet in chlorine’s valence shell. It needs one electron to make it stable at 8 electrons in its valence shells. This makes chlorine a #cl^(−1)#.
from socratic.org
Chlorine has 7 valence electrons. This makes chlorine a #cl^(−1)#. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. (in table salt, this electron comes from the sodium atom.) e− + cl cl− e − + cl cl −. The 3s^2 3p^5 electrons are the outermost electrons, so chlorine has seven valence electrons. It needs one electron to make it stable at 8 electrons in its valence shells. Valence electrons = 7, lone pair. Valence electrons are the electrons in the outermost shell, or energy level, of an atom. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Only one more electron is needed to achieve an octet in chlorine’s valence shell.
Chlorine combined with two negative atom or 1 positive and other
Chlorine Valence Electrons Charge In a picture, the valence. It needs one electron to make it stable at 8 electrons in its valence shells. The 3s^2 3p^5 electrons are the outermost electrons, so chlorine has seven valence electrons. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. This makes chlorine a #cl^(−1)#. Only one more electron is needed to achieve an octet in chlorine’s valence shell. Chlorine has 7 valence electrons. Valence electrons = 7, lone pair. In a picture, the valence. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. (in table salt, this electron comes from the sodium atom.) e− + cl cl− e − + cl cl −. Valence electrons are the electrons in the outermost shell, or energy level, of an atom.
From www.klipartz.com
Lewis structure Chlorine Valence electron Diagram, 18, miscellaneous Chlorine Valence Electrons Charge In a picture, the valence. It needs one electron to make it stable at 8 electrons in its valence shells. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. Valence electrons are the electrons in the outermost shell, or energy level, of an atom. Only one more electron. Chlorine Valence Electrons Charge.
From chemistry291.blogspot.com
What Is the Chlorine(Cl) Electron Configuration? Chlorine Valence Electrons Charge (in table salt, this electron comes from the sodium atom.) e− + cl cl− e − + cl cl −. Valence electrons are the electrons in the outermost shell, or energy level, of an atom. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Only. Chlorine Valence Electrons Charge.
From topblogtenz.com
Chlorine Orbital diagram, Electron configuration, and Valence electrons Chlorine Valence Electrons Charge Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. Only one more electron is needed to achieve an octet in chlorine’s valence shell. The 3s^2 3p^5 electrons are the outermost electrons, so chlorine has seven valence electrons. It needs one electron to make it stable at 8 electrons. Chlorine Valence Electrons Charge.
From www.numerade.com
SOLVED (a) Describe the molecule chlorine dioxide, ClO2, using three Chlorine Valence Electrons Charge Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. Only one more electron is needed to achieve an octet in chlorine’s valence shell. It needs one electron to make it stable at 8 electrons in its valence shells. In a picture, the valence. 93 rows you may assume. Chlorine Valence Electrons Charge.
From courses.lumenlearning.com
Electrons Biology for Majors I Chlorine Valence Electrons Charge 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. It needs one electron to make it stable at 8 electrons in its valence shells. Only one more electron is needed to achieve an octet in chlorine’s valence shell. The 3s^2 3p^5 electrons are the outermost. Chlorine Valence Electrons Charge.
From chemistry291.blogspot.com
How Many Valence Electrons Does chlorine Have?number of valence Chlorine Valence Electrons Charge This makes chlorine a #cl^(−1)#. Only one more electron is needed to achieve an octet in chlorine’s valence shell. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. It needs one electron to make it stable at 8 electrons in its valence shells. Chlorine has. Chlorine Valence Electrons Charge.
From valenceelectrons.com
How to Find the Valence Electrons for ClF3 (Chlorine Trifluoride)? Chlorine Valence Electrons Charge 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. The 3s^2 3p^5 electrons are the outermost electrons, so chlorine has seven valence electrons. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. Valence. Chlorine Valence Electrons Charge.
From www.crowlex.com
How Many Valence Electrons Does Chlorine Have Crowlex Chlorine Valence Electrons Charge Valence electrons = 7, lone pair. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. Chlorine has 7 valence electrons. It needs one electron to make it stable at 8 electrons in its valence shells. 93 rows you may assume the valences of the chemical elements—the number of. Chlorine Valence Electrons Charge.
From chamotgallery.com
How many protons, neutrons and electrons does chlorine have? (2023) Chlorine Valence Electrons Charge Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. The 3s^2 3p^5 electrons are the outermost electrons, so chlorine has seven valence electrons. Chlorine has 7 valence electrons. It needs one electron to make it stable at 8 electrons in its valence shells. Only one more electron is. Chlorine Valence Electrons Charge.
From material-properties.org
Chlorine Periodic Table and Atomic Properties Chlorine Valence Electrons Charge Valence electrons = 7, lone pair. Chlorine has 7 valence electrons. It needs one electron to make it stable at 8 electrons in its valence shells. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. The 3s^2 3p^5 electrons are the outermost electrons, so chlorine. Chlorine Valence Electrons Charge.
From valenceelectrons.com
How Many Valence Electrons Does ClO2 and ClO2 Have? Chlorine Valence Electrons Charge Valence electrons = 7, lone pair. Chlorine has 7 valence electrons. It needs one electron to make it stable at 8 electrons in its valence shells. This makes chlorine a #cl^(−1)#. Valence electrons are the electrons in the outermost shell, or energy level, of an atom. The 3s^2 3p^5 electrons are the outermost electrons, so chlorine has seven valence electrons.. Chlorine Valence Electrons Charge.
From www.sciencephoto.com
Chlorine, atomic structure Stock Image C018/3698 Science Photo Library Chlorine Valence Electrons Charge Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Chlorine has 7 valence electrons. Only one more electron is needed to achieve an octet. Chlorine Valence Electrons Charge.
From valenceelectrons.com
How to Find the Valence Electrons for PCl3? Chlorine Valence Electrons Charge Only one more electron is needed to achieve an octet in chlorine’s valence shell. In a picture, the valence. Chlorine has 7 valence electrons. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. The 3s^2 3p^5 electrons are the outermost electrons, so chlorine has seven. Chlorine Valence Electrons Charge.
From socratic.org
What is the Lewis electrondot structure for a molecule of hydrogen Chlorine Valence Electrons Charge Valence electrons are the electrons in the outermost shell, or energy level, of an atom. Only one more electron is needed to achieve an octet in chlorine’s valence shell. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. 93 rows you may assume the valences of the chemical. Chlorine Valence Electrons Charge.
From www.hiclipart.com
Free download Chemistry, Atom, Bohr Model, Electron, Chlorine Chlorine Valence Electrons Charge 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Valence electrons are the electrons in the outermost shell, or energy level, of an atom. The 3s^2 3p^5 electrons are the outermost electrons, so chlorine has seven valence electrons. In a picture, the valence. Some atoms. Chlorine Valence Electrons Charge.
From www.animalia-life.club
Electron Configuration For Chlorine Chlorine Valence Electrons Charge In a picture, the valence. (in table salt, this electron comes from the sodium atom.) e− + cl cl− e − + cl cl −. Chlorine has 7 valence electrons. The 3s^2 3p^5 electrons are the outermost electrons, so chlorine has seven valence electrons. Valence electrons = 7, lone pair. Valence electrons are the electrons in the outermost shell, or. Chlorine Valence Electrons Charge.
From exatin.info
Electron Dot Diagram For Chlorine exatin.info Chlorine Valence Electrons Charge Only one more electron is needed to achieve an octet in chlorine’s valence shell. Valence electrons are the electrons in the outermost shell, or energy level, of an atom. (in table salt, this electron comes from the sodium atom.) e− + cl cl− e − + cl cl −. It needs one electron to make it stable at 8 electrons. Chlorine Valence Electrons Charge.
From www.alamy.com
Chlorine atomic structure Cut Out Stock Images & Pictures Alamy Chlorine Valence Electrons Charge Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. Valence electrons = 7, lone pair. Chlorine has 7 valence electrons. Only one more electron is needed to achieve an octet in chlorine’s valence shell. (in table salt, this electron comes from the sodium atom.) e− + cl cl−. Chlorine Valence Electrons Charge.